Arthrosamid®is a new type of treatment for knee osteoarthritis that offers patients an effective alternative to the currently available therapies.1 Based on an innovative non-biodegradable hydrogel technology, Arthrosamid® is 2.5% cross-linked polyacrylamide and 97.5% water. When injected into the knee Arthrosamid® cushions the joint and reduces pain, providing safe and sustained relief, all with one injection.1
Knee Osteoarthritis?
Take the next step in controlling your pain
What is osteoarthritis?
Osteoarthritis is a long term condition which affects the joints and worsens over time, causing pain and disability. It is most common in the knee joint,² where it wears away the shock-absorbing cartilage, causing bones to rub together and the joint to become stiff, swollen and painful. Around 1 in 5 people aged over 45 in England is affected by knee osteoarthritis.3
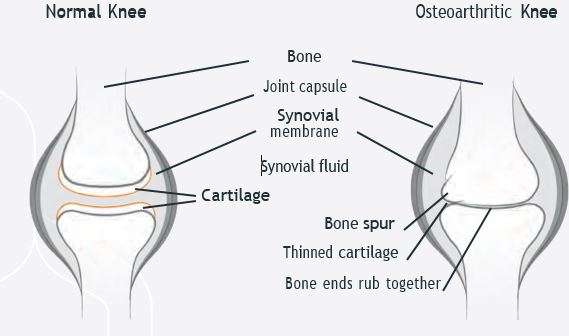
The cause of osteoarthritis is unknown. There is no permanent cure and, until now, treatments have focused on managing the symptoms. They include:
- Exercise
- Weight loss
- Pain relief medication
(including prescription-only opioids and off-label corticosteroid injections)
- Knee injections with viscosupplements
- Surgery such as joint osteotomy or knee replacement

Apart from knee replacement surgery,
these therapies cannot deliver long-term benefits.
How is Arthrosamid® administered?
Arthrosamid® is given as a single injection into the knee’s intra-articular cavity by a qualified specialist – either an orthopaedic surgeon or rheumatologist. Before you are given an Arthrosamid® injection, you will have a local anaesthetic, which may sting a little, to numb the area around your knee. Unlike surgery, the injection is a minimally invasive procedure that you can receive as an outpatient. Before the injection, you should be given antibiotics to protect you from any potential risk of infection.
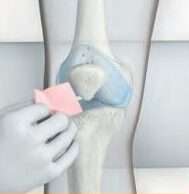
Step 1
The knee is cleaned prior to the Arthrosamid® injection.5

Step 2
The injection of Arthrosamid® is performed with the help of ultrasound.5
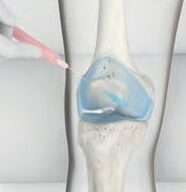
Step 3
Arthrosamid® is injected using a needle.5
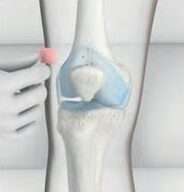
Step 4
The needle is removed and a plaster is placed over the injection site.
What should I expect immediately after the procedure?1
– Following the procedure, you may be allowed to leave immediately, or you may wish to rest until you feel ready to go.
– Your knee may start to feel uncomfortable as the anaesthetic wears off, but many patients do not report any discomfort.
– If you do feel discomfort, symptoms may include mild to moderate pain and/or swelling at the injection site. If your knee becomes red, hot, largely swollen or more painful, you should contact the injecting physician immediately for treatment.
– You should continue your regular medication and pain killers as normal.
– Arthrosamid® may feel like a different sensation to other injections that you have previously experienced as it is more viscous than other injectable treatments.
How does it work?
Arthrosamid® is an intra-articular polyacrylamide hydrogel injection for the symptomatic treatment of knee osteoarthritis. Arthrosamid® also integrates into the synovium of the inner joint capsule creating a cushion-like effect.
What are the benefits?
As Arthrosamid® works to cushion the joint, it can reduce your pain, decrease stiffness, and help movement.1,6-9 The hydrogel itself does not degrade and therefore it provides long-acting relief, improving your quality of life.¹ In clinical trials, patients reported a significant reduction in their pain symptoms by week 4 after their injection and, unlike other injectable treatments, the average level of reduction was maintained at the 3-year follow up period.10
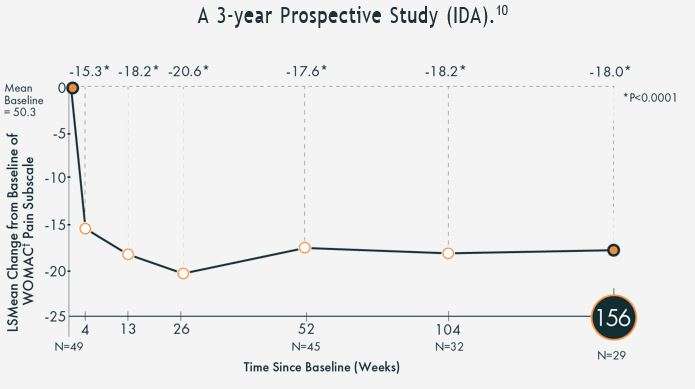
† WOMAC or The Western Ontario and McMaster Universities Osteoarthritis Index is a measure of symptoms and physical disability. LSMeans are modelled/estimated means. The estimated means are using data from the other visits and also the covariates.
When will I begin to feel less pain?
You are likely to feel some pain relief within 24 hours; however, it can take 4 to 12 weeks to feel the maximum benefit. Clinical data shows that most patients start to feel an effect within a few weeks.1
When can I go back to normal activity?
You should avoid any strenuous weight-bearing activities (e.g. running, tennis or long walks) during the first few days after your injection. Your doctor will be able to advise you on how to slowly introduce more activity.
Do I need to tell any healthcare providers about my treatment?
If in the future you require a major surgical or dental procedure, you should tell your treating physician that you have an Arthrosamid® implant in your knee to ensure an accurate medical assessment.
How long will the treatment last?
The hydrogel making up the injection will not degrade, and therefore will provide long-lasting relief. So far, most patients have reported a significant reduction in their pain 4 weeks after their injection, and the average level of reduction was maintained at the 3-year follow-up period.5
What are the side effects?
In a number of clinical trials with Arthrosamid®, there were no serious side effects related to the hydrogel. The most commonly reported side effects were mild to moderate injection site pain and swelling, which were generally transient in nature. The overall safety profile of the hydrogel has been established over the last 20 years with its use for various indications in the body.
Is Arthrosamid® right for me?
Arthrosamid® is approved for the symptomatic treatment of knee osteoarthritis so any patient with this condition may be suitable. Over the last 10 years many patients have benefitted from treatment with the hydrogel in Arthrosamid®. However treatment with Arthrosamid® may not be suitable for everyone. Your doctor is the best person to advise you, but situations where you should not use Arthrosamid® (contraindications) include*:
- If you have an infection at or near the injection site
- If you have haemophilia or take anticoagulant treatment
- If you have had a knee arthroscopy within the past 6 months
Additionally, your doctor may advise you not to have treatment with Arthrosamid® if your diabetes is poorly controlled, you are having major dental work, or you have been diagnosed with an autoimmune disorder, (such as Multiple Sclerosis, Addison’s, or Coeliac disease). If you are under 18, pregnant, breastfeeding, or have a foreign body in your knee, your doctor may advise against the injection. This is because the safety and effectiveness of Arthrosamid® has not yet been established in these groups.
How does Arthrosamid® differ from other injectable treatments?
Arthrosamid® is different from the viscosupplement injections for the knee such as hyaluronic acid (HA), platelet rich plasma (PRP) and
regenerative stem cell therapy. Unlike these treatments, Arthrosamid® is a non degradable hydrogel which means it has the properties to lubricate and cushion for longer. Unlike the other treatments, Arthrosamid® also has the ability to become an integrated part of the soft synovial tissue in the joint capsule which may lead to longer lasting pain relief.5
Take the next step in controlling your knee OA pain
Knee osteoarthritis can be a debilitating condition impairing your quality of life. Over-the-counter painkillers or lifestyle changes to diet and exercise are often not enough. Talk to your doctor about Arthrosamid®.
More information is available from
Info@thearmdoc.co.uk
or
020 3384 5588
INDICATIONS, PATIENT GROUP AND USAGE
Arthrosamid® is intended to be used for symptomatic treatment
of adult patients with knee osteoarthritis.
CONTRAINDICATIONS5
Arthrosamid® should not be injected:
– If an active skin disease or infection is present at or near the injection site
– If the joint is infected or severely inflamed
– If the patient has previously received treatment with a different non-absorbable injectable/implant – If the patient has received a knee alloplasty or has any foreign material in the knee
– If the patient has undergone knee arthroscopy within the last 6 months
– In haemophilia patients or in patients in uncontrolled anti-coagulant treatment
1. Bliddal H, Overgaard A, Hartkopp A, Beier J, Conaghan PG, et al. (2021) Polyacrylamide Hydrogel Injection for Knee Osteoarthritis: A 6 Months Prospective Study. J Orthop Res Ther 6: 1188.
2. Jorge L. Escobar Ivirico, Maumita Bhattacharjee, Emmanuel Kuyinu, Lakshmi S. Nair, Cato T. Laurencin, Regenerative Engineering for Knee Osteoarthritis Treatment: Biomaterials and Cell-Based Technologies, Engineering, Volume 3, Issue 1,2017, P16-27.
3. Public Health England. Available at: https://www.versusarthritis.org/media/13374/birmingham-oa-1.pdf. Accessed 23 03 2021.
4. Goldman, D.T., Piechowiak, R., Nissman, D., Bagla, S., Isaacson, A., 2018. Current Concepts and Future Directions of Minimally Invasive Treatment for Knee Pain. Current rheumatology reports 20, 54.
5. Arthrosamid, Instructions For Use. Release Date March 2022 10083-003.
6. Christensen, L., Daugaard, S., 2016. Histological Appearance of the Synovial Membrane after Treatment of Knee Osteoarthritis with Polyacrylamide Gel Injections: A Case Report. Journal of Arthritis 5, 217.
7. Tnibar, A., Schougaard, H., Camitz, L., Rasmussen, J., Koene, M., Jahn, W., Markussen, B., 2015. An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up. Acta veterinaria Scandinavica 57, 20.
8. Christensen, L., Camitz, L., Illigen, K.E., Hansen, M., Sarvaa, R., Conaghan, P.G., 2016. Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthritis and cartilage / 24, 1999-2002.
9. Data on file.
10. Henriksen, M., et al. (2023) 3 Year Results From a Prospective Study of Polyacrylamide Hydrogel for Knee Osteoarthritis. Poster 483 presented at OARSI 2023.
OUS/ARTHRO/MAR2023/048
References:
1. Bliddal H, Overgaard A, Hartkopp A, Beier J, Conaghan PG, et al. (2021) Polyacrylamide Hydrogel Injection for Knee Osteoarthritis: A 6 Months Prospective Study. J Orthop Res Ther 6: 1188.
2. Christensen, L., Daugaard, S., 2016. Histological Appearance of the Synovial Membrane after Treatment of Knee Osteoarthritis with Polyacrylamide Gel Injections: A Case Report. Journal of Arthritis 5, 217.
3. Tnibar, A., Schougaard, H., Camitz, L., Rasmussen, J., Koene, M., Jahn, W., Markussen, B., 2015. An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24
months follow-up. Acta veterinaria Scandinavica 57, 20.
4. Goldman, D.T., Piechowiak, R., Nissman, D., Bagla, S., Isaacson, A., 2018. Current Concepts and Future Directions of Minimally Invasive Treatment for Knee Pain. Current rheumatology reports 20, 54.
5. Henriksen, M., et al. (2023) 3 Year Results From a Prospective Study of Polyacrylamide Hydrogel for Knee Osteoarthritis. Poster 483 presented at OARSI 2023.
OUS/ARTHRO/MAR2023/049



